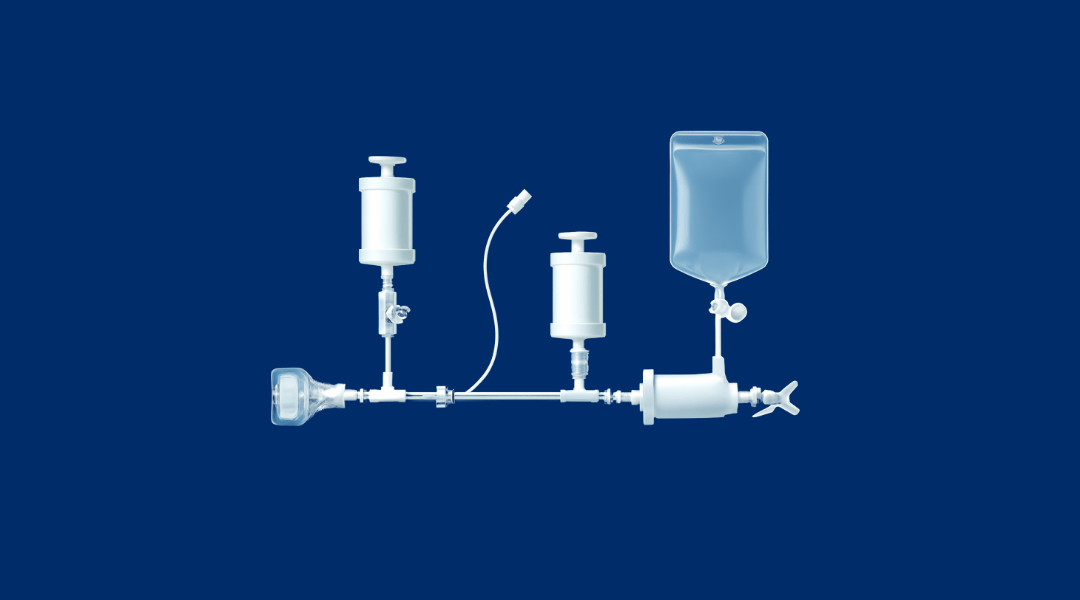
What is PUPSIT (Pre-Use/Post Sterilisation Integrity Testing)?
PUPSIT (Pre-Use Post Sterilisation Integrity Testing) is a test performed to ensure the integrity of sterilising filters and system components such as tubing, connectors, and more. It checks for any potential damage or loss of integrity that may occur during the filter setup before use. PUPSIT is a European regulatory requirement for sterile medicinal products. First introduced in Annex 1 in 1996 and further emphasised by the EMA in its 2008 update, PUPSIT is a mandatory step in the final filling process, unless proven technically unfeasible.
Without PUPSIT, minor filter damage from transport or installation may remain undetected. Testing the integrity after sterilisation but before use ensures the system is safe for operation. PUPSIT is a critical element of the updated EU GMP Annex 1 guidelines.
Global Regulations for Integrity Testing
Different international guidelines prescribe requirements for integrity testing of sterilising-grade filters:
-
EU GMP: Integrity of the sterilised filter must be verified before use and confirmed after use using appropriate methods, such as the Bubble Point test or Diffusion Flow test.
-
US FDA: Integrity testing can be performed prior to processing but is required after use.
-
NMPA (China): Filter integrity must be confirmed and documented immediately after use using suitable methods.
-
GMP Guidelines: Integrity testing before and after use is essential for sterile assurance.
-
PIC/S 2017: Integrity must be confirmed both before use and immediately after use using accepted methods.
| Guideline/Authority | Pre-Sterilisation | Post-Sterilisation | Pre-Use | Post-Use |
|---|---|---|---|---|
| EMEA/PIC/S | - | Required | - | Required |
| NMPA | Testable | Testable | - | Required |
| FDA | Testable | Testable | - | Required |
| WHO (cGMP) | Testable | Testable | - | Required |
How to perform PUPSIT?
- Important considerations when performing PUPSIT:Maintain sterility on the downstream side.
- Add extra components to the filter setup, such as hydrophobic sterilising filters (to control bioburden), sterile collection containers, or single-use bags for liquid or gas disposal.
Van Borselen Filters provides all components required for effective PUPSIT implementation.
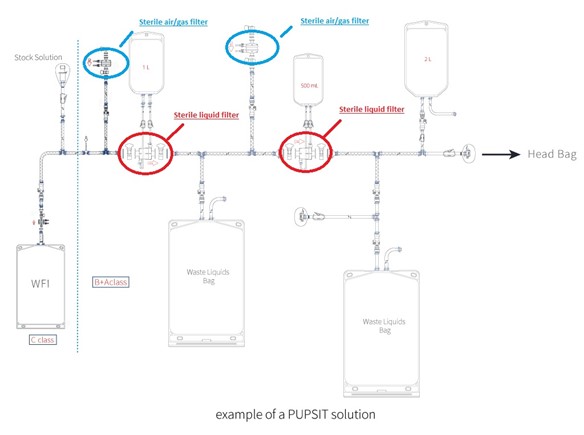
At Van Borselen Filters, we offer everything needed to perform PUPSIT effectively.
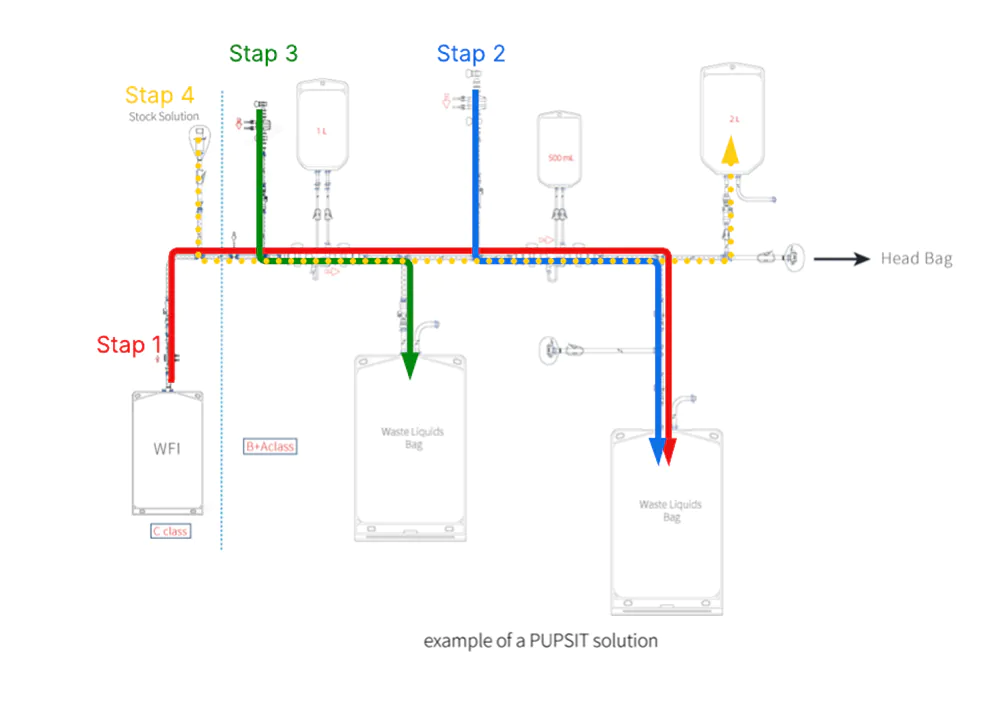
Step 1: Pre-wetting the sterile liquid filters
Ensure that sufficient Water for Injection (WFI) flows through both sterile liquid filters to properly pre-wet them. Collect the WFI in a sterile single-use bag.
Step 2: Integrity test of the first sterile liquid filter
Connect a sterile air filter to the system to ensure the test gas remains sterile. Collect the test gas in a single-use bag. If the filter passes the test, proceed to step 3.
Step 3: Integrity test of the second sterile liquid filter
Again, ensure that a sterile air filter is connected to the system, so the test gas remains sterile. Collect the gas in a single-use bag.
Step 4: Filter the stock solution
Direct the stock solution through both validated filters into a sterile single-use bag. Take a sample from the filtered liquid. If the results are approved, the system is ready for sterile filtration of the stock solution into the head bag for final filling.
Integrity Testing Methods:
- Bubble Point Test: Measures the pressure required to force gas through wetted pores. If continuous gas flow occurs below the specified bubble point, the filter has failed. If the flow starts at or above the specified value, the filter passes.
- Diffusion or Forward Flow Test: Measures gas permeability at a fixed pressure (below bubble point). For example, a manufacturer may specify ≤18 ml/min per 10” module at 1800 mbar. If flow remains within this limit, the filter passes; if exceeded, the filter fails. This method is especially suited for single-use systems.
Some manufacturers use both methods for extra assurance, though this is not mandatory.
Benefits of PUPSIT
PUPSIT helps detect filter defects before use, increasing safety during sterile medicine production. Advocates argue that existing controls alone may miss minor faults, making PUPSIT critical for patient safety.
At Van Borselen Filters, we offer user-friendly, fully in-line PUPSIT systems—eliminating relocation, external testing, and contamination risk. Our systems are designed to test filter integrity both before and after sterilisation.
Learn more? Do you have any questions or would you like more information? Please fill in your details below, and our team will get in touch with you shortly. We are happy to assist you!
Contact us