
Case
API filters in the pharmaceuticals
Read this blog about Active Pharmaceutical Ingredients (APIs), the active substances in pharmaceutical products.
Read moreCheckout using your account
Checkout as a new customer
Creating an account has many benefits:

Case
Read this blog about Active Pharmaceutical Ingredients (APIs), the active substances in pharmaceutical products.
Read more
News
Sterile filtration removes microorganisms without affecting product quality; only 0.2 µm filters that retain 10⁷ CFU of Brevundimonas diminuta in accordance with ASTM F838 are considered sterile.
Read more
Case
Reliable gas filtration for compressed air and nitrogen removes oil, moisture and particles, ensuring sterile, controlled gas streams for GMP-compliant pharmaceutical and veterinary production.
Read more
News
This blog explains differences between absolute and nominal filters, efficiency, selection, and why filters and integrity testing are essential for sterile pharma and biotech.
Read more
News
Porous sintered metal delivers reliable filtration under extreme temperatures, pressure and chemicals, combining durability and consistent performance for demanding industrial applications.
Read more
News
Chocolate is more expensive than ever. That’s why it’s essential to prevent waste. With the right screening technology, you can easily recover off-spec product and increase your yield – even as a small-scale chocolatier. Discover how SWECO helps you get more out of your chocolate production process.
Read more
News
Filtratie is onmisbaar in elke stap van het kaasproductieproces – van wei- en luchtfiltratie tot het zuiveren van water en zoutbaden. In deze blog leest u welke filtratietechnieken er zijn en welke oplossingen Van Borselen Filters hiervoor biedt.
Read more
News
PUPSIT (Pre-Use/Post Sterilisation Integrity Testing) is essential for ensuring the integrity of sterilizing filters in the biotechnology and pharmaceutical industries. In this blog, we explain what PUPSIT is, how it works, and the benefits it offers for maintaining sterility assurance in your processes.
Read more
News
This blog helps evaluate whether reusing sterile filters is cost-effective, highlighting risks, validation requirements, hidden costs and viable alternatives for reliable sterile filtration.
Read more
Case
Read more about the impact of excess endotoxins on the drug manufacturing process.
Read more
Case
Filtrox has been our reliable partner in depth filtration for decades, with a focus on filter sheets and modules.
Read more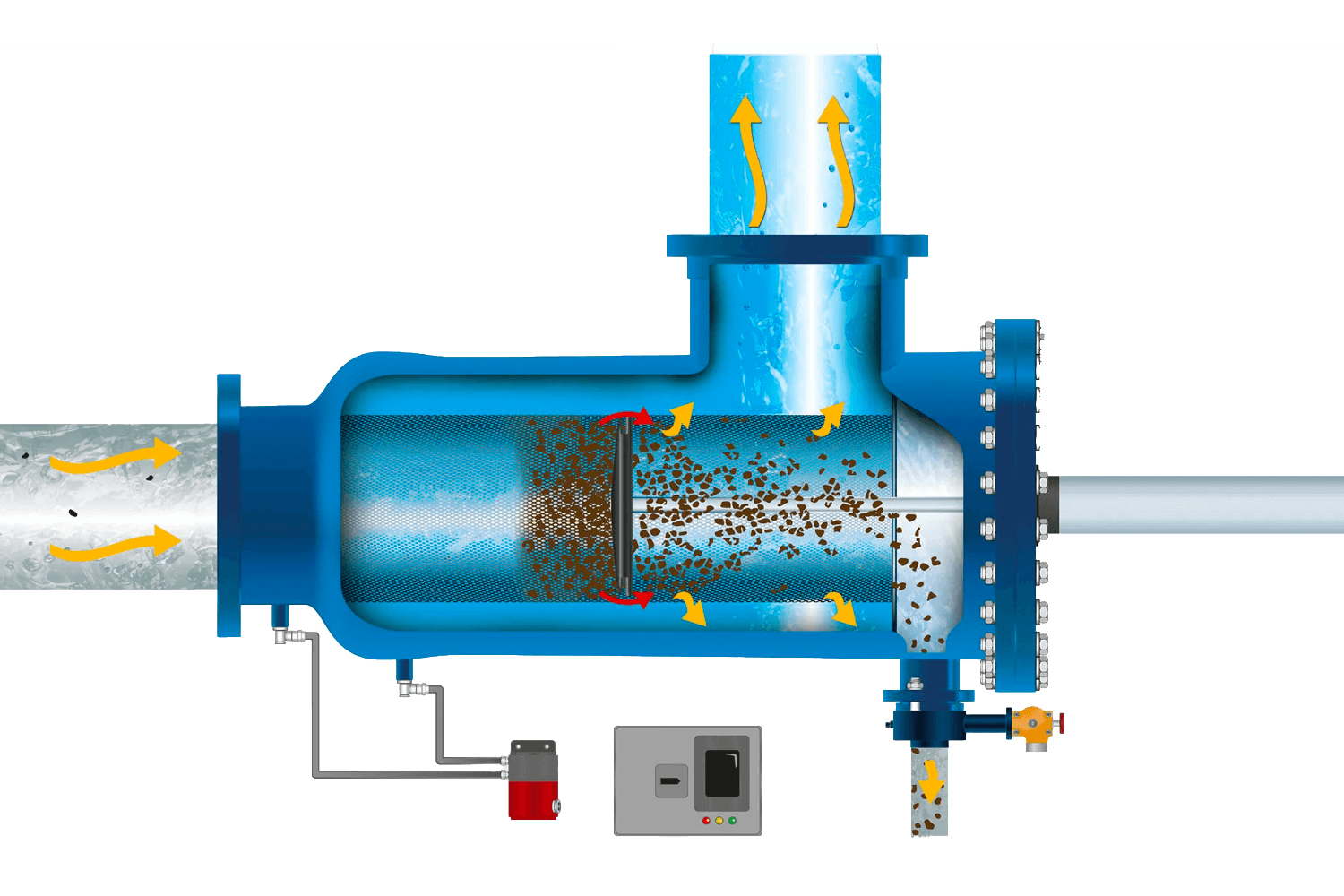
News
It's important to protect your heat exchangers to ensure its' maximum lifespan. In this blog you'll learn which filters can help protect heat exchangers and you'll read about a success story of Van Borselen Filters.
Read more